BBMRI-CZ – website
BBMRI-CZ – website
Hosting institution: Masaryk Memorial Cancer Institute
Partner institutions:
- First Medical Faculty, Charles University
- Medical Faculty, Charles University in Hradec Králové
- Medical Faculty, Charles University in Pilsen
- Medical Faculty, Palacký University in Olomouc
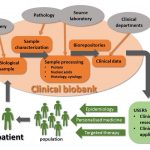
The research infrastructure BBMRI-CZ collects, processes and stores human-derived biospecimens (such as primary tumor tissues and other relevant human-derived specimens) that would be otherwise irreversibly lost. These biospecimens are collected using standardized procedures and are of critical importance for existing or future clinical and translational research leading to potential patient benefit as well. In the Czech Republic, BBMRI-CZ organizes not only a dedicated set of cancer-oriented biorepositories but also operates a unique set of technologies and knowledge to perform clinical applications of translational research including clinical trials. The user community takes advantage of expertise of the BBMRI-CZ qualified staff and resources archived in biorepositories. BBMRI-CZ represents a Czech National Node of the pan-European research infrastructure BBMRI (Biobanking and Biomolecular Resources Research Infrastructure) and has been a Member State of the BBMRI ERIC since 2013.
Budoucí rozvoj
In academic area, the BBMRI-CZ development plan encompasses taking leadership in the field of research-oriented clinical biobanking in the Czech Republic including setting up a network of regional biobanks to focus on premorbid period in cancer in the context of regional exposure in the Czech Republic. At the academia-industry interface BBMRI-CZ will increase its role as a leading partner for innovative industrial activities to enhance introduction of new potential medicinal and/or diagnostic products to better serve patient community in the Czech Republic.
Socioekonomické přínosy
Direct socio-economic impacts of BBMRI-CZ pertain to activities defining key documents of health policies in the Czech Republic such as clinical practice guidelines on the use of clinical laboratory and predictive testing in oncology. Indirect impacts pertain to the medical applications of biomarkers to be discovered and characterized with the use of collected biological material connected to clinical data and tested through a comprehensive system of clinical trials. Search for relevant biomarkers specific for certain disease using archived human tissues is a critical component in the design of innovative medicinal products and diagnostic procedures in human disease.